In a newly published study, we describe our design for a self-extinguishing rechargeable battery. It replaces the most commonly used electrolyte, which is highly combustible – a medium composed of a lithium salt and an organic solvent – with materials found in a commercial fire extinguisher.
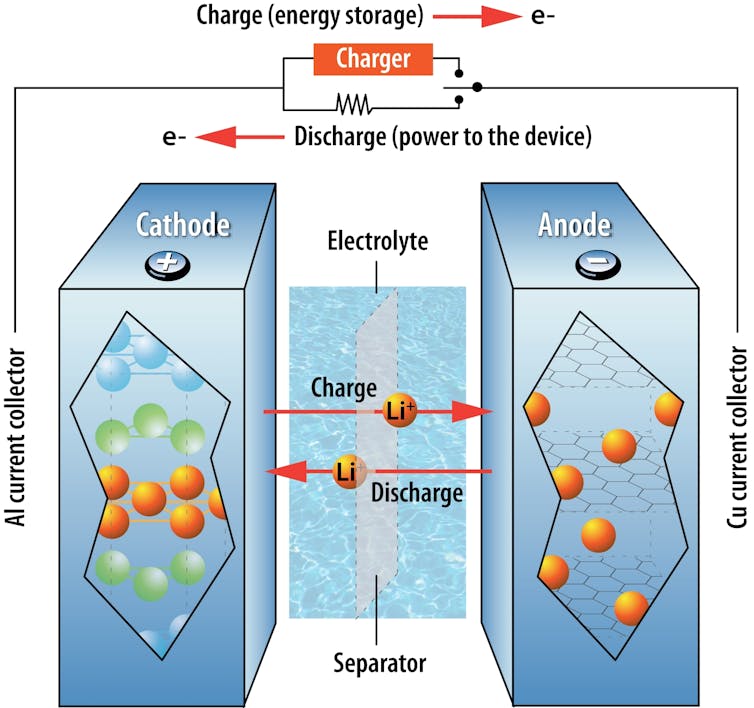
An electrolyte allows lithium ions that carry an electric charge to move across a separator between the positive and negative terminals of a lithium-ion battery. By modifying affordable commercial coolants to function as battery electrolytes, we were able to produce a battery that puts out its own fire.
Our electrolyte worked well across a wide temperature range, from about minus 100 to 175 degrees Fahrenheit (minus 75 to 80 degrees Celsius). Batteries that we produced in the lab with this electrolyte transferred heat away from the battery very well, and extinguished internal fires effectively.
We subjected these batteries to the nail penetration test, a common method for assessing lithium-ion battery safety. Driving a stainless steel nail through a charged battery simulates an internal short circuit; if the battery catches fire, it fails the test. When we drove a nail through our charged batteries, they withstood the impact without catching fire.
Why it matters
By nature, a battery’s temperature changes as it charges and discharges, due to internal resistance – opposition within the battery to the flow of lithium ions. High outdoor temperatures or uneven temperatures within a battery pack seriously threaten batteries’ safety and durability.
Energy-dense batteries, such as the lithium-ion versions that are widely used in electronics and electric vehicles, contain an electrolyte formulation dominated by organic molecules that are highly flammable. This worsens the risk of thermal runaway – an uncontrollable process in which excess heat inside a battery speeds up unwanted chemical reactions that release more heat, triggering further reactions. Temperatures inside the battery can rise by hundreds of degrees in a second, causing a fire or explosion.
Another safety concern arises when lithium-ion batteries are charged too quickly. This can cause chemical reactions that produce very sharp lithium needles called dendrites on the battery’s anode – the electrode with a negative charge. Eventually, the needles penetrate the separator and reach the other electrode, short-circuiting the battery internally and leading to overheating.
As scientists studying energy generation, storage and conversion, we have a strong interest in developing energy-dense and safe batteries. Replacing flammable electrolytes with a flame-retardant electrolyte has the potential to make lithium-ion batteries safer, and can buy time for longer-term improvements that reduce inherent risks of overheating and thermal runaway.
How we did our work
We wanted to develop an electrolyte that was nonflammable, would readily transfer heat away from the battery pack, could function over a wide temperature range, was very durable, and would be compatible with any battery chemistry. However, most known nonflammable organic solvents contain fluorine and phosphorus, which are expensive and can have harmful effects on the environment.
Instead, we focused on adapting affordable commercial coolants that already were widely used in fire extinguishers, electronic testing and cleaning applications, so that they could function as battery electrolytes.
We focused on a mature, safe and affordable commercial fluid called Novec 7300, which has low toxicity, is nonflammable and does not contribute to global warming. By combining this fluid with several other chemicals that added durability, we were able to produce an electrolyte that had the features we sought and would enable a battery to charge and discharge over a full year without losing significant capacity.
What still isn’t known
Because lithium – an alkali metal - is scarce in our Earth’s crust, it is important to investigate how well batteries that use other, more abundant alkali metal ions, such as potassium or sodium, fare in comparison. For this reason, our study focused predominantly on self-extinguishing potassium-ion batteries, although it also showed that our electrolyte works well for making self-extinguishing lithium-ion batteries.
It remains to be seen whether our electrolyte can work equally well for other types of batteries that are in development, such as sodium-ion, aluminum-ion and zinc-ion batteries. Our goal is to develop practical, environmentally friendly, sustainable batteries regardless of their ion type.
For now, however, since our alternative electrolyte has similar physical properties to currently used electrolytes, it can be readily integrated with current battery production lines. If the industry embraces it, we expect that companies will be able to manufacture nonflammable batteries using their existing lithium-ion battery facilities.
The Research Brief is a short take on interesting academic work.![]()
Apparao Rao, Professor of Physics, Clemson University and Bingan Lu, Associate Professor of Physics and Electronics, Hunan University
This article is republished from The Conversation under a Creative Commons license. Read the original article.














