Rechargeable batteries are great for storing energy and powering electronics, from smartphones to electric vehicles. However, they can be more difficult to charge in cold environments and may even catch on fire.
I’m a mechanical engineering professor who has been interested in batteries since college. I now lead a battery research group at Drexel University.
In just this past decade, I have watched the price of lithium-ion batteries drop as the production market has grown significantly. Future projections predict the market could dramatically increase by thousands of GWh annually by 2030.
However, lithium-ion batteries aren’t perfect, and their rise comes with risks, such as their tendency to slow down during cold weather and even catch on fire.
Behind the Li-ion battery
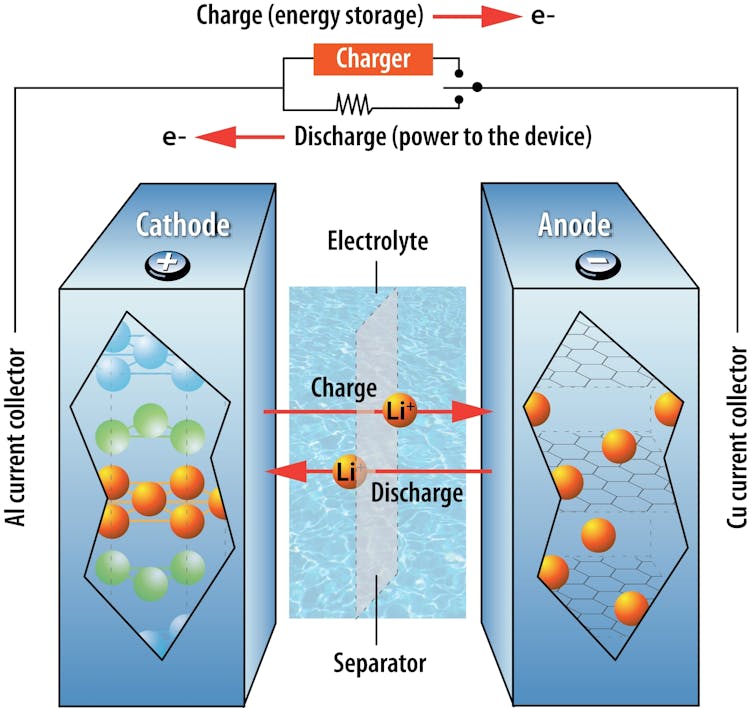
The electrochemical energy storage within batteries works by storing electricity in the form of ions. Ions are atoms with a nonzero charge because they have too many or insufficient electrons.
When you plug in your electric car or phone, the electricity provided by the outlet drives these ions from the battery’s positive electrode into its negative electrode. The electrodes are solid materials in a battery that can store ions, and all batteries have both a positive and a negative electrode.
Electrons pass through the battery as electricity. With each electron that passes to one electrode, a lithium-ion also passes into the same electrode, ensuring the balance of charges in the battery. As you drive your car, the stored ions in the negative electrode move back to the positive electrode, and the resulting flow of electricity powers the motor.
While AA or AAA batteries can power small electronics, they can only be used once and cannot be charged. Rechargeable Li-ion batteries can operate for thousands of cycles of full charge and discharge, and each cycle can also store a much higher charge than an AA or AAA battery.
Since lithium is the lightest metal, it has a high specific capacity and can store considerable charge per weight. This is why lithium-ion batteries are helpful not just for portable electronics but for powering modes of transportation with limited weight or volume, such as electric cars.
Battery fires
However, lithium-ion batteries have risks that AA or AAA batteries don’t. For one, they’re more likely to catch on fire. In the past five years, the number of electric bike battery fires reported in New York City has increased from 30 to nearly 300.
Lots of different issues can cause a battery fire. Poorly manufactured cells could contain defects, such as trace impurities or particles left behind from the manufacturing process, that increase the risk of internal failure.

Climate can also affect battery operation. Electric vehicle sales have increased across the U.S., particularly in cold regions such as the Northeast and Midwest, where the frigid temperatures can hinder battery performance.
Batteries contain fluids called electrolytes, and cold temperatures cause fluids to flow more slowly. So, the electrolytes in batteries slow and thicken in the cold, causing the lithium ions inside to move slower. This slowdown can prevent the lithium ions from correctly inserting into the electrodes. Instead, they may deposit on the electrode surface and form lithium metal.
Too much lithium deposits on the electrode’s surface during charging may cause an internal short circuit. This process can start a battery fire.
Making safer batteries
My research group and many others are studying how to make more efficient batteries in the cold.
For example, researchers are exploring swapping out the usual battery electrolyte for an alternative that doesn’t thicken at cold temperatures. Another potential option is heating the battery pack before charging so that the charging process occurs at a warmer temperature.
My group is also investigating new types of batteries beyond lithium-ion. These could include batteries that are more stable at broader temperature ranges, those that don’t use liquid electrolytes, or those that use sodium instead of lithium. Abundant sodium-ion batteries could work well and cost less.
Solid-state batteries use solid electrolytes that aren’t flammable, reducing the fire risk. However, they don’t work as well as Li-ion batteries, so more research is needed to determine whether they are a good option.
Lithium-ion batteries power technologies that people across the country use every day, and research in these areas aims to find solutions that will make this technology even safer for the consumer.
Wesley Chang, Assistant Professor of Mechanical Engineering and Mechanics, Drexel University
This article is republished from The Conversation under a Creative Commons license. Read the original article.














